For decades, Ocean Iron Fertilization (OIF) has been both celebrated and feared — viewed by some as a groundbreaking way to capture carbon and restore marine life, and by others as a potential ecological gamble.
But what if many of these fears were based on misunderstandings rather than evidence?
Today, after years of global research, data paints a very different picture: responsible ocean fertilization is not pollution — it’s restoration.
Let’s clear the air and separate the myths from the science.
Misconception 1: “It will trigger toxic red tides and kill marine life.”
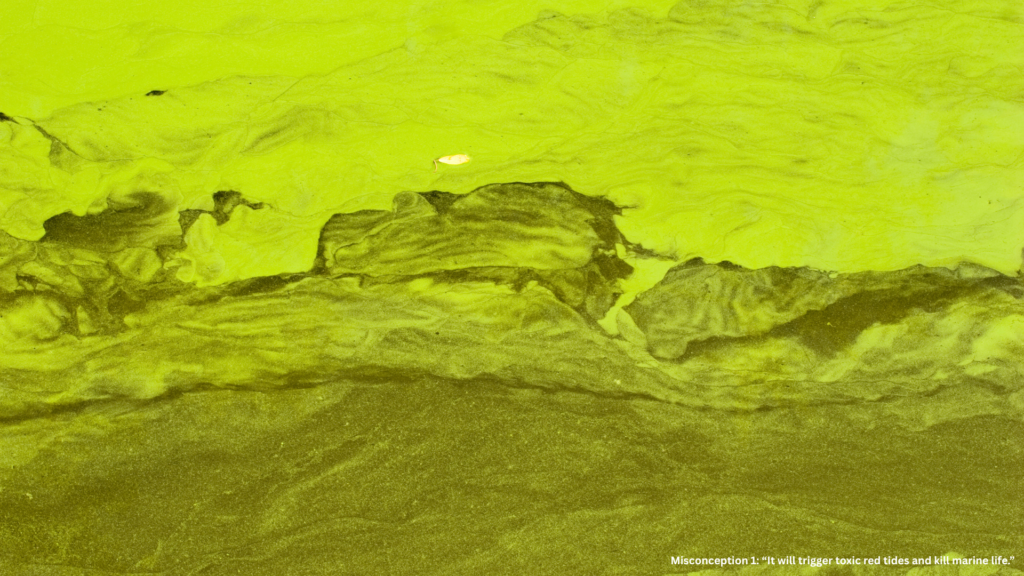
This misconception stems from mixing up two very different things — open-ocean fertilization and coastal eutrophication.
In polluted coastal waters, nutrient overloads (from sewage or fertilizers) can create harmful algal blooms that suffocate marine life.
Ocean Iron Fertilization, however, works in the exact opposite context: the open ocean — places rich in nutrients but poor in iron, known as High Nutrient, Low Chlorophyll (HNLC) zones.
Here’s the key difference:
- OIF adds only trace amounts of iron, measured in parts per trillion — millions of times lower than toxic levels.
- The ocean areas are vast and well-mixed, so algae don’t accumulate excessively.
- All major OIF experiments — IronEx II, SOFeX, SEEDS, EIFEX, and LOHAFEX — showed healthy, balanced plankton blooms, dominated by beneficial diatoms and grazed by zooplankton.
- No red tides.
- No dead fish.
- No oxygen depletion.
Even when species like Pseudo-nitzschia appeared (known to produce toxins in coastal waters), no toxins were found under open-ocean conditions.
In other words, OIF doesn’t poison ecosystems — it revives them.
Misconception 2: “It will create oxygen-depleted dead zones.”

Dead zones happen when excess organic matter decays in enclosed waters, consuming oxygen faster than it’s replenished — a problem common in coastal bays and estuaries, not in the open ocean.
The open ocean is a different story. It’s vast, turbulent, and oxygen-rich due to constant mixing and contact with the atmosphere. In every OIF study so far, scientists have recorded no oxygen depletion during or after fertilization.
In fact, there’s evidence that OIF could actually reduce the formation of coastal dead zones.
How? By capturing excess nutrients in the Southern Ocean before they drift northward and fuel oxygen-starved areas like those near Peru or Namibia.
So instead of creating dead zones, responsible OIF could help prevent them.
Misconception 3: “It steals nutrients that other fisheries depend on.”
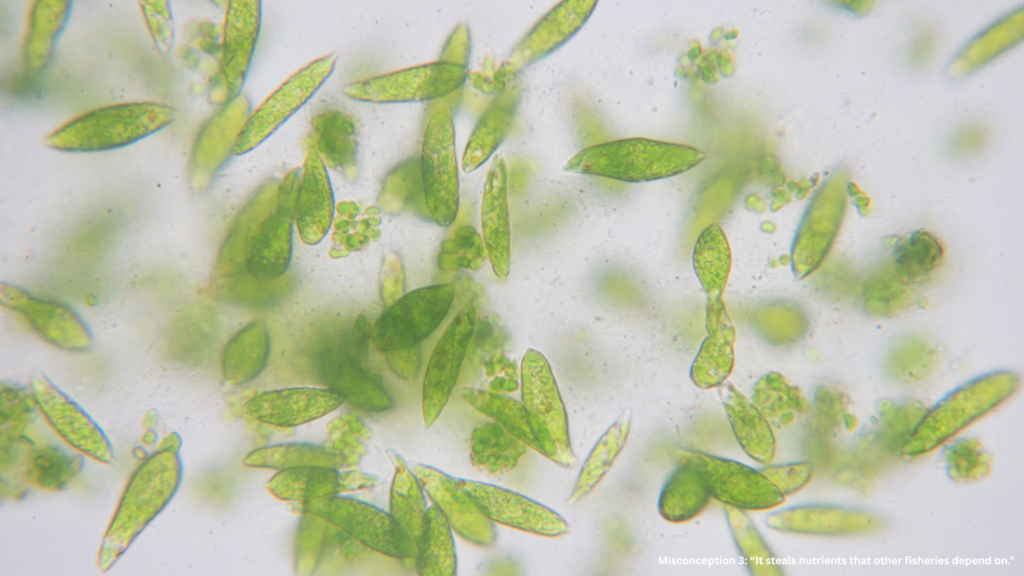
It’s easy to imagine the ocean as a pantry — use nutrients in one place, and another goes hungry. But the ocean doesn’t work like that.
The global nutrient cycle is a slow-moving conveyor belt that recycles elements over decades.
OIF doesn’t take nutrients away from fisheries — it activates nutrients that were already going unused.
For example, large areas of the Southern Ocean are overflowing with nitrate and phosphate, but plankton can’t use them because of iron scarcity. Adding a trace of iron there unlocks that productivity, transforming “wasted” nutrients into food for marine life.
Moreover:
- Not all nutrients are ever fully consumed — other factors like sunlight and grazing limit growth.
- Responsible OIF focuses on inefficient regions, far from major fishing grounds.
The result? More plankton, more fish food, and more carbon storage.
That’s a win for marine ecosystems and fisheries.
Misconception 4: “Iron fertilization could poison the ocean.”

Iron isn’t a pollutant — it’s an essential nutrient. Life in the ocean literally depends on it.
The iron added during OIF is:
- In microscopic quantities, comparable to natural inputs from wind-blown dust or volcanic ash.
- Rapidly bound by organic compounds and absorbed by plankton.
- Harmless to fish, corals, and marine mammals — every major OIF experiment confirmed thriving biodiversity.
In fact, the ocean naturally receives thousands of times more iron each year from natural sources.
OIF simply gives a gentle, targeted boost — in the right place, at the right time.
Concern 5: “We don’t know the long-term effects.”
This is a fair and important point — large-scale OIF has never been done before, and long-term monitoring is essential.
But what’s often overlooked is that climate change itself is already altering ocean ecosystems — warming, acidification, and oxygen loss are happening everywhere. Compared to these rapid disruptions, controlled, well-monitored OIF is a small and manageable intervention.
After 30 years of research, no OIF experiment has shown lasting ecosystem disruption. Blooms fade naturally after weeks, species composition remains balanced, and the system resets.
Global policy bodies like the London Protocol and Convention on Biological Diversity now support regulated OIF trials — a sign that science, not speculation, is guiding the next phase.
Moving Forward: From Fear to Knowledge
The truth is simple:
Most of the fears that halted OIF were never backed by data. No red tides. No dead zones. No toxic oceans.
What we have instead is a powerful, nature-based solution that can:
- Absorb atmospheric carbon,
- Revitalize marine food webs,
- Improve global fisheries, and
- Stabilize the climate system.
The challenge now isn’t whether OIF is safe — it’s how to scale it responsibly, with transparency, monitoring, and global cooperation.
In a time when the planet needs every tool we can responsibly deploy, Ocean Fertilization offers hope — not hazard.
Final Thought
The ocean has always been Earth’s greatest healer.
All we’re doing with iron fertilization is returning a missing ingredient, allowing it to keep healing — for us, and for the generations to come.
 WhatsApp
WhatsApp  Book A Meeeting
Book A Meeeting